Sterile Powder Filling Machine
BRIEF INTRODUCTION
Standalone Machine Positioning
Designed to independently perform aseptic filling / stoppering / crimping operations, fully meeting GMP requirements for standalone aseptic production.
Applicable Packaging & Specifications
Container type: Vials (tubular glass vials)
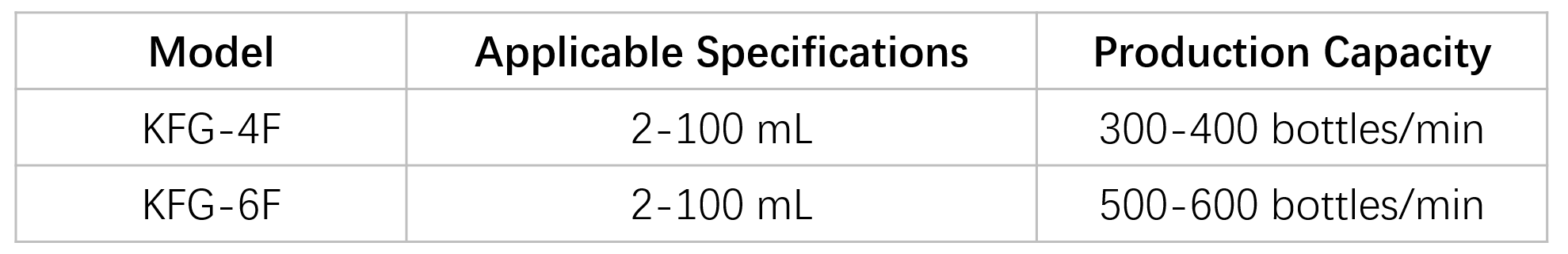
Filling range: 2 – 100 mL
Output: 9,000 vials/hour
(Measured under operating conditions of 10 mL vials with powder products)
Process Flow
Bottle unscrambling → In-machine operations: online air blowing + plasma dust removal → A-grade laminar airflow hood with magnetic levitation positioning → servo-driven screw-type aseptic filling → Vacuum stoppering → servo-controlled crimping → Online check weighing → Bottle discharge
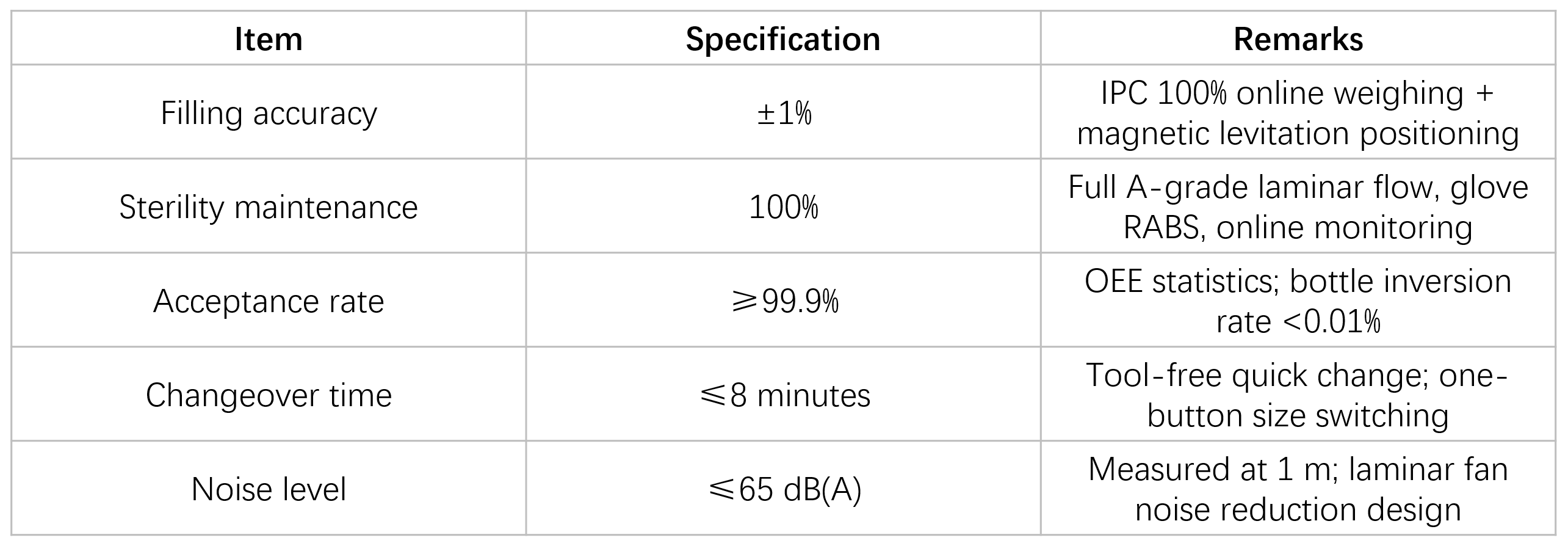
Key Performance Parameters
Structure & GMP Design
Fully enclosed 316 stainless steel frame, electropolished to Ra ≤0.4 μm, featuring zero dead corners and no exposed screws.
Top-mounted ISO Class 5 (Grade A) laminar airflow hood with airflow velocity of 0.35–0.45 m/s; side-mounted RABS glove box enables isolated operation.
All interfaces use quick-clamp connections, supporting online CIP/SIP and VHP (vaporized hydrogen peroxide) sterilization.
The bottle discharge area is equipped with a continuous bag-out system, preventing disruption of the Grade A environment.
Control System
Beckhoff TwinCAT PLC combined with a Siemens 15" color HMI, supporting ≥100 recipe sets.
Full compliance with 21 CFR Part 11, including audit trails, electronic signatures, PDF batch records, and USB/Ethernet data export.
Real-time monitoring of particle counts, airflow velocity, and differential pressure, with seamless integration to OPC UA / MES / LIMS systems.
Utility Requirements



GIVE US A MESSAGE