Oral Liquid Filling Line
BRIEF INTRODUCTION
Product Positioning
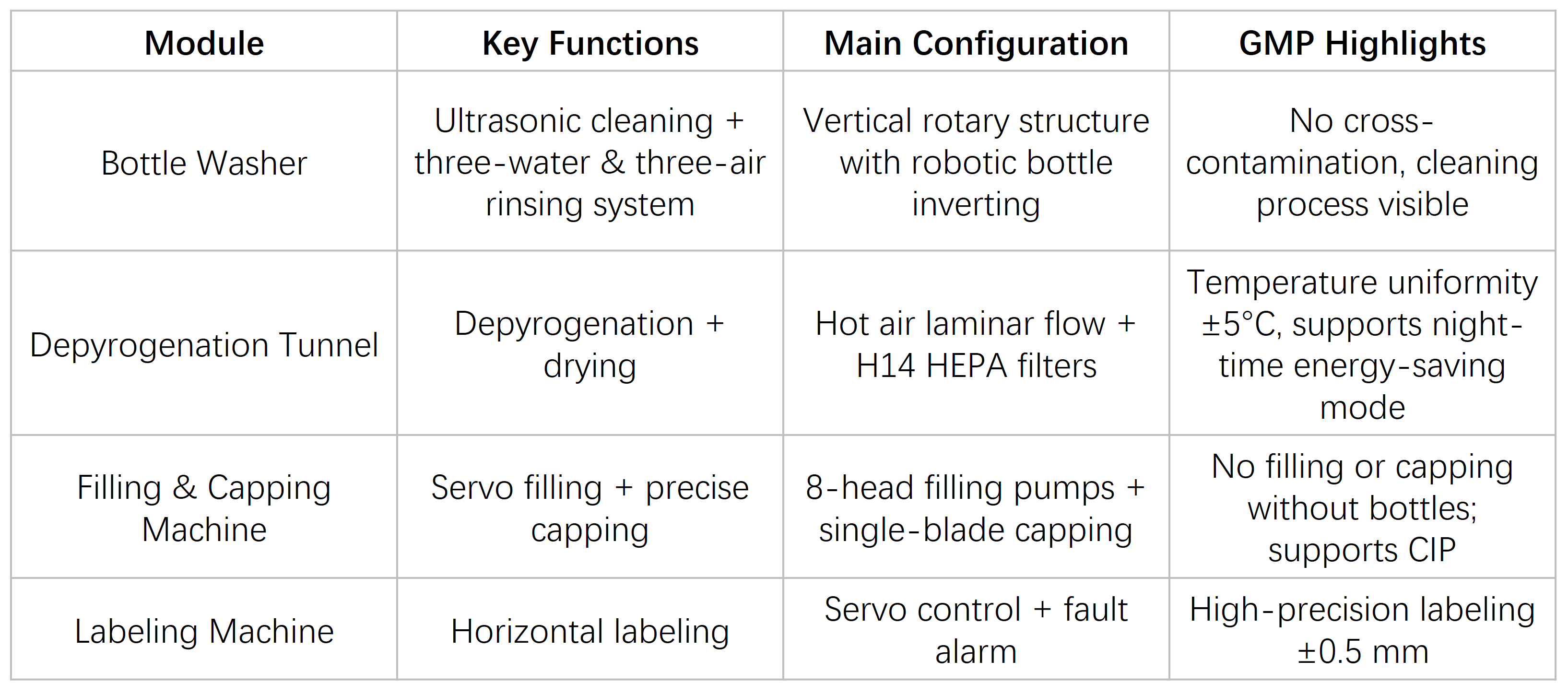
Designed to meet GMP-compliant production requirements for pharmaceuticals such as oral liquid preparations and biological products, covering the entire process of vial washing / sterilization / filling / stoppering / labeling.
Applicable Packaging Type & Specifications
Container type: 10 mL glass vials with rubber stopper and aluminum cap
Filling volume range: 2 – 50 mL (standard: 10 mL)
Maximum output: 12,000 vials/hour
(Measured under operating conditions of 10 mL glass oral liquid vials filled with aqueous solution)
Process Flow
Vial infeed → Washing: ultrasonic + three-water / three-air process, high-temperature WFI at 60–65 °C → Sterilization: dry heat depyrogenation, 250 °C × 15 min, air velocity 0.7–0.8 m/s → Filling: servo-driven piston pump, accuracy ≤ ±1% → Stoppering / Capping: stopper & cap placement accuracy ≥ 99% → Capping: qualified rate ≥ 99%, with door interlock protection → Labeling: labeling rate ≥ 99%, labeling accuracy ±0.5 mm
Core Modules
Control System & Data Integrity
Siemens / Mitsubishi PLC with HMI, supporting emergency stop, safety door interlock, and audible & visual alarms; one-button CIP start.
Full-line sensor monitoring (missing vial, full vial, no vial–no fill, no vial–no cap).
Compliant with 21 CFR Part 11, including audit trail, automatic batch record generation, and one-key recipe changeover (≥ 100 recipes).
Cleaning & Sterilization Strategy
Filling system supports automatic CIP/SIP, ensuring high online cleaning efficiency.
Dry heat depyrogenation tunnel: 250 °C × 15 min, optional high-efficiency mode: 300 °C × 5 min.
Online filter integrity testing; positive-pressure standby mode for energy-saving operation during night shifts.
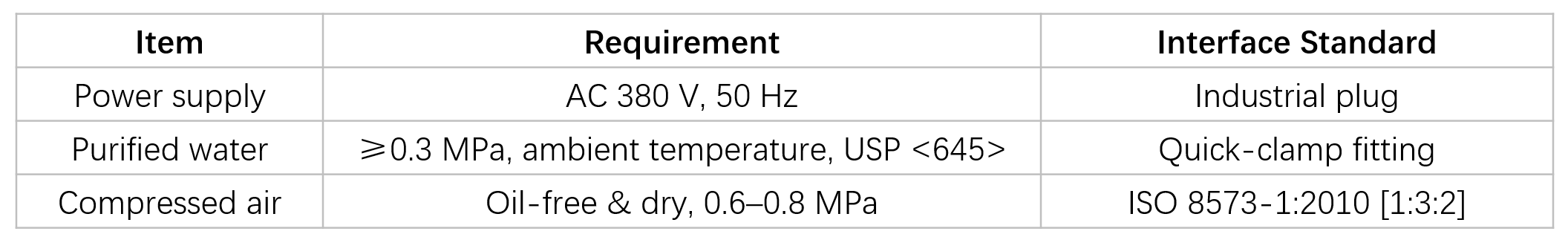
Utility Requirements
JKQXL Vertical Ultrasonic Washing Machine

JKRX Series Hot Air Circulation and Sterilizing Tunnel

JKKF-24 Oral Liquid Filling and Capping Machine

GIVE US A MESSAGE