Infusion Bottle Filling Line
BRIEF INTRODUCTION
Product Positioning
Designed to meet GMP-grade production requirements for sterile pharmaceutical products, covering the complete process of bottle washing → sterilization → filling → stoppering/capping → labeling.
Applicable Packaging Type & Specifications
Packaging Type: Infusion bottles
Fill Volume Range: 50 – 500 mL
Maximum Output: Up to 6,000 bottles/hour
(verified under 100 mL glass infusion bottles, liquid product conditions)
Process Flow
Bottle infeed → Ultrasonic washing (two-water / one-air, 50–100 bottles/min) → Dry heat sterilization (hot air 300 °C × 5 min) → Filling (servo-driven with peristaltic pump, ±0.5% accuracy) → Stoppering / capping (acceptance rate ≥99.9%) → Vertical labeling (accuracy ±1 mm)
Core Modules
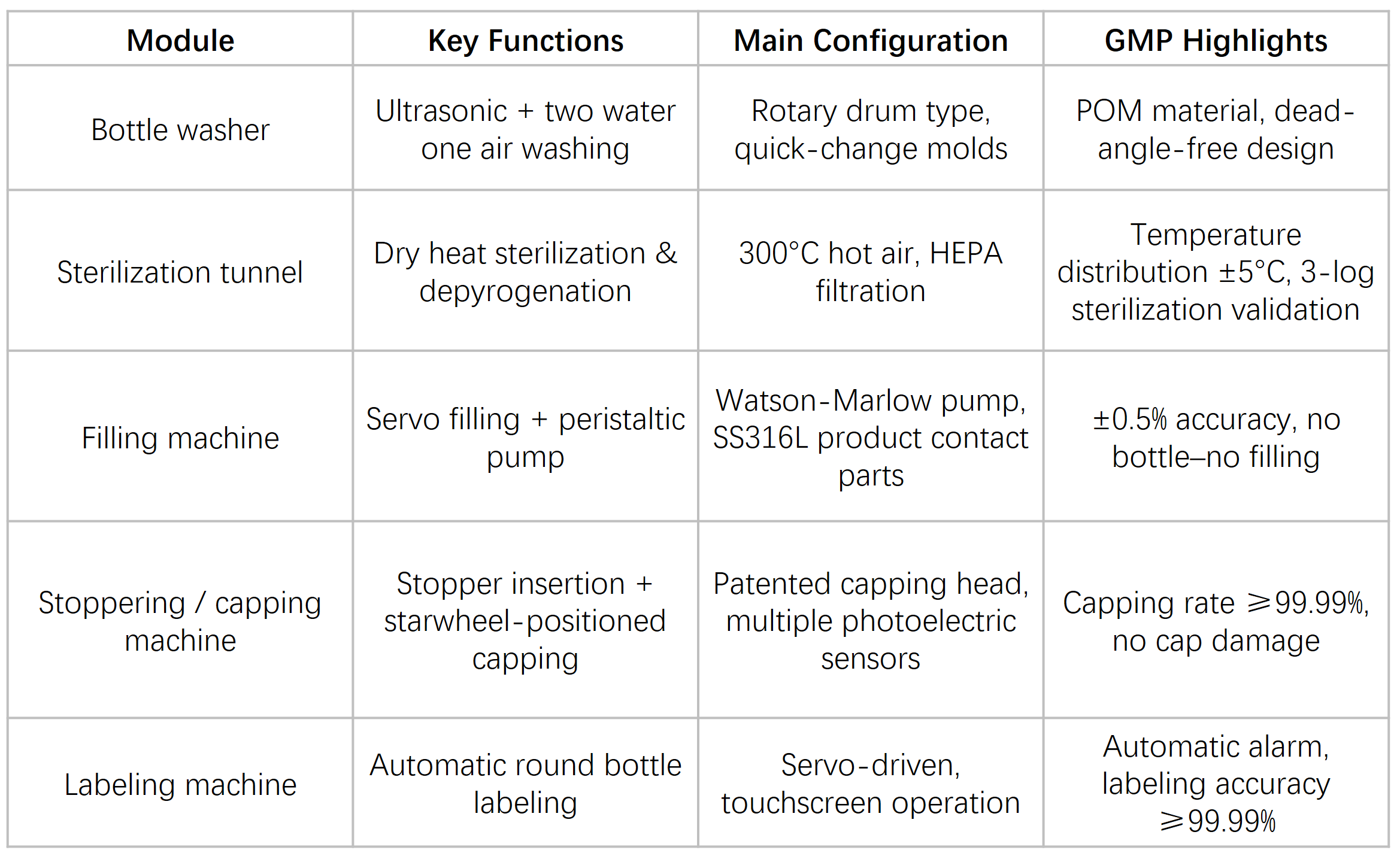
Control System & Data Integrity
The entire line is controlled by a Siemens PLC with an industrial touch-screen HMI, supporting user access management and fast recipe changeover.
All operating data are monitored in real time with interlinked alarms, and electronic batch records can be generated automatically.
The system supports MES integration and complies with 21 CFR Part 11 data integrity requirements.
Washing & Sterilization Strategy
The washing area supports interchangeable molds, with laser-cut drum structures for rapid format change
Sterilization tunnel options: dry heat 300 °C × 15 min or 250 °C × 30 min, achieving endotoxin reduction ≥3 log
Cooling zone airflow of 0.6–0.7 m/s, with an automatic overpressure balancing system to ensure cleanroom stability
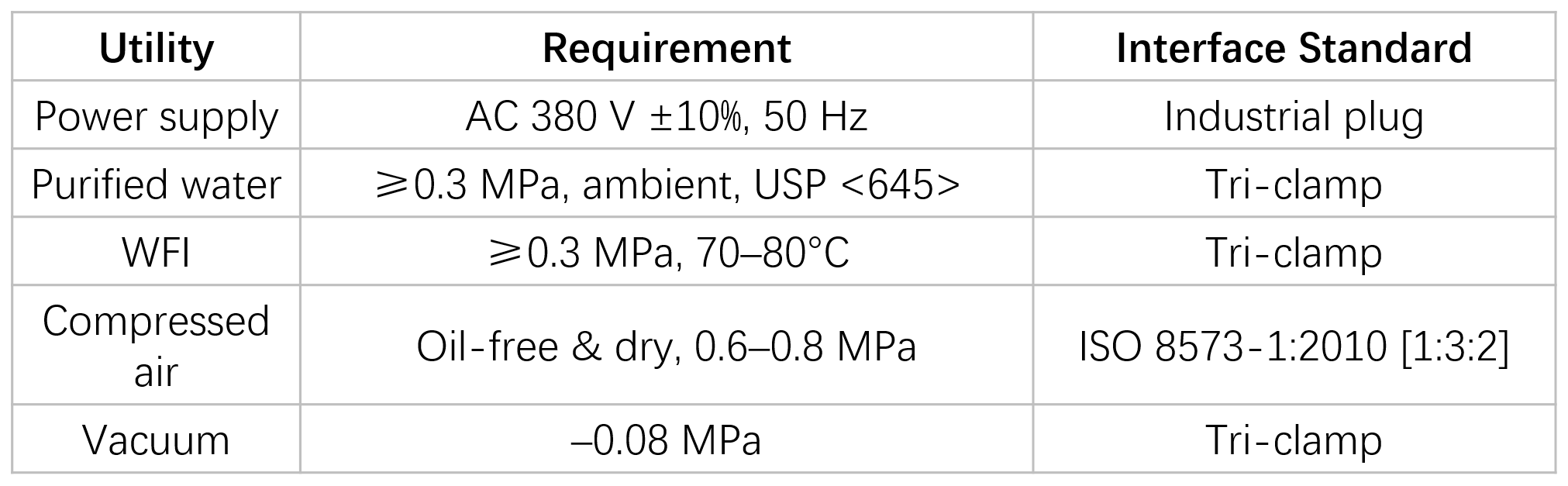
Utility Requirements
JKGP Series Turntable

JKQCX Rotary Cylinder Ultrasonic Washing Machine

JKRX Hot Air Circulating Heating and Sterilizing Tunnel

JKGS Filling and Stoppering Machine

JKZ Star Wheel Capping Machine

GIVE US A MESSAGE









