Diagnostic Reagent Filling Line
BRIEF INTRODUCTION
Product Positioning
Designed to meet GMP-compliant production requirements for products such as in vitro diagnostic (IVD) reagents, biochemical reagents, immunological products, covering the entire process of bottle unscrambling / air rinsing / filling / capping / discharge.
Applicable Packaging Types & Specifications
Container types: Round plastic bottles, vials, cartridge bottles, test tubes, ophthalmic dropper bottles
Filling volume range: 2 – 500 mL
Maximum output: 4,800 bottles/hour
(Measured under operating conditions of 10 mL plastic bottles filled with aqueous solution)
Process Flow
Manual bottle loading → Bottle unscrambling: turntable + elevator → Air rinsing: clean compressed air, 0.5 MPa × 3 s → Filling: servo-driven peristaltic pump, accuracy ±1% → Cap sorting: vibrating bowl + vacuum cap pick-up → Servo capping: adjustable torque 0.3–1.2 N·m → Bottle discharge: automatic robotic unloading → Optional: vision inspection + labeling + cartoning
Core Modules
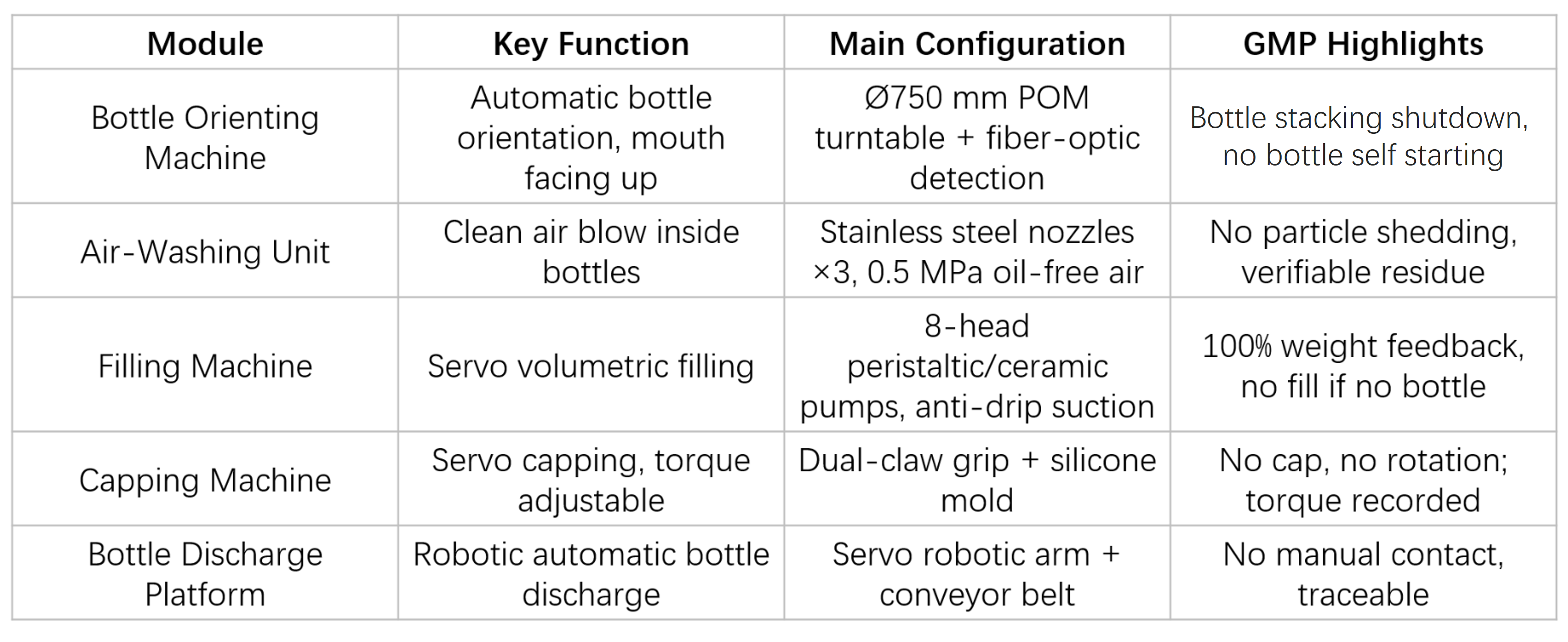
Control System & Data Integrity
Siemens PLC + Siemens 10" touchscreen HMI, three-level user access control, 21 CFR Part 11–compliant audit trail.
Recipe management for ≥100 product formats, with one-click changeover.
Automatic batch record generation with electronic signatures, USB/PDF export, and OPC UA interface for MES integration.
Cleaning & Sterilization Strategy
Whole-machine CIP: 304 stainless steel quick-release structure, online circulation cleaning with coverage ≥ 99%.
Peristaltic / ceramic pumps: offline SIP (121 °C × 30 minutes).
Air rinsing nozzles are detachable for cleaning; compressed air filters support online integrity testing.
Utility Requirements

GIVE US A MESSAGE




