BFS Line
BRIEF INTRODUCTION
Standalone Machine Positioning
The machine independently completes aseptic blow molding, filling, and sealing processes, meeting GMP requirements for standalone aseptic production.
Applicable Packaging & Specifications
Packaging Type: Plastic PE / PP aseptic bottles
Aseptic Bottle Volume Range: 5 mL, 10 mL
Maximum Output: Up to 6,000 bottles/hour
Mold Size: Single mold with 40 cavities
Module Configuration: 4 sets × 10 cavities
Process Flow
Material feeding → In-machine processes: plastic pellet heating and extrusion → bottle blowing and forming → filling → sealing → cutting → bottle discharge
Key Performance Parameters
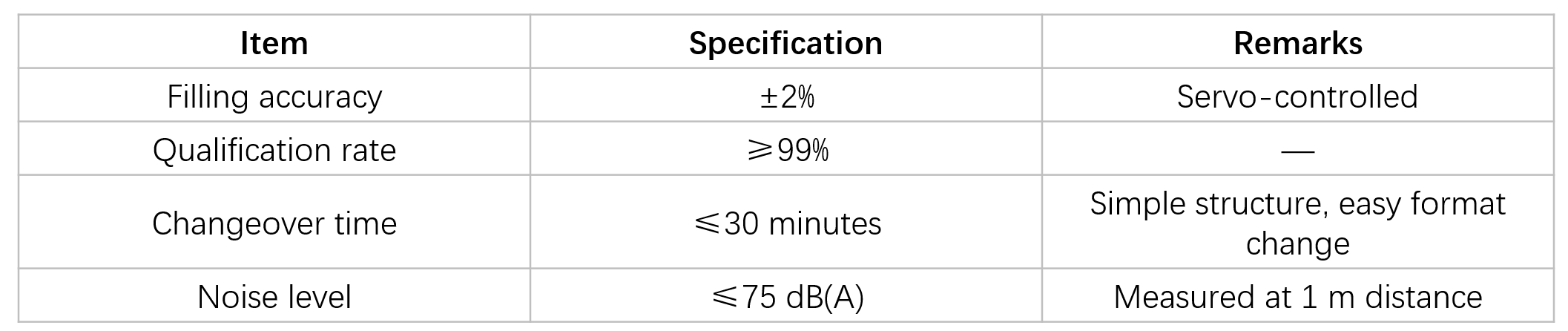
Structure & GMP Design
Extrusion, forming, filling, and sealing are completed under aseptic conditions within an integrated system, minimizing personnel, environmental, and material contamination, and ensuring product safety.
Pharmaceutical-grade plastic resins are used, featuring excellent heat resistance and low permeability to steam and air, and complying with EU GMP and USP standards.
The machine supports CIP (Clean-in-Place) and SIP (Sterilize-in-Place), ensuring equipment cleanliness and sterility in compliance with GMP requirements.
Control System
An advanced electronic control system equipped with Schneider or ABB touch-screen HMI, offering an intuitive interface for easy parameter setting and monitoring.
High-precision control components are utilized, including ratio pressure controllers, optocoupler relays, miniature relays, valve terminals, servo drives, and servo motors.
Multiple safety protection devices—such as overload thermal relays, motor protectors, and safety door interlocks—are provided to ensure operational safety and stable machine performance.
All production steps—including heating, extrusion, blowing, forming, filling, and sealing—are fully automated to minimize manual intervention.
Utility Requirements
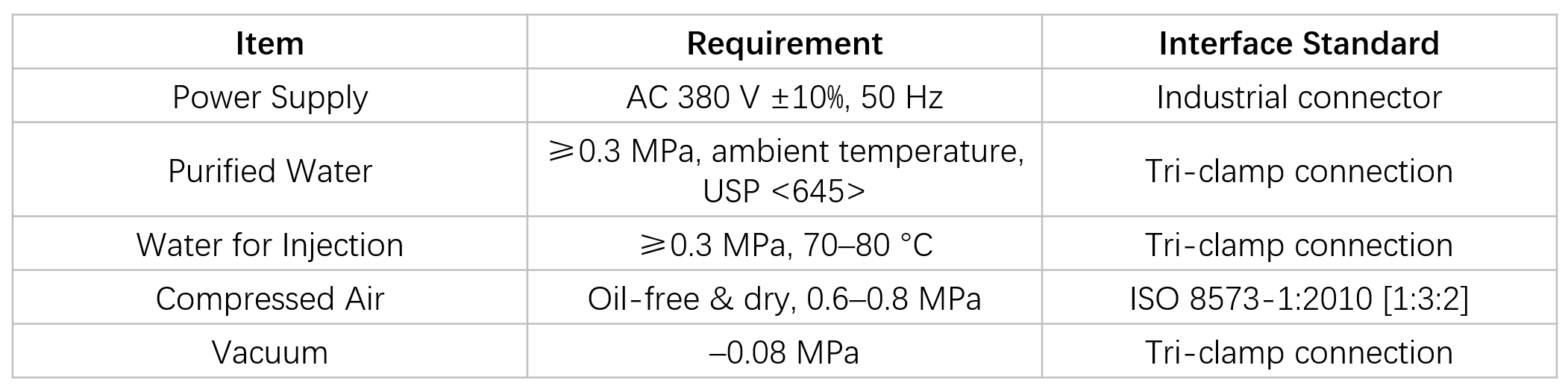
GIVE US A MESSAGE





