Plastic Bottle Syrup Filling Line
BRIEF INTRODUCTION
Product Positioning
Designed to meet GMP-grade production requirements for oral liquid pharmaceuticals and biological products, covering the complete process of filling / capping / labeling / cartoning.
Applicable Packaging Type & Specifications
Packaging Type: PET bottles / glass bottles / oral liquid bottles
Fill Volume Range: 20 – 500 mL
Maximum Output: Up to 12,000 bottles/hour
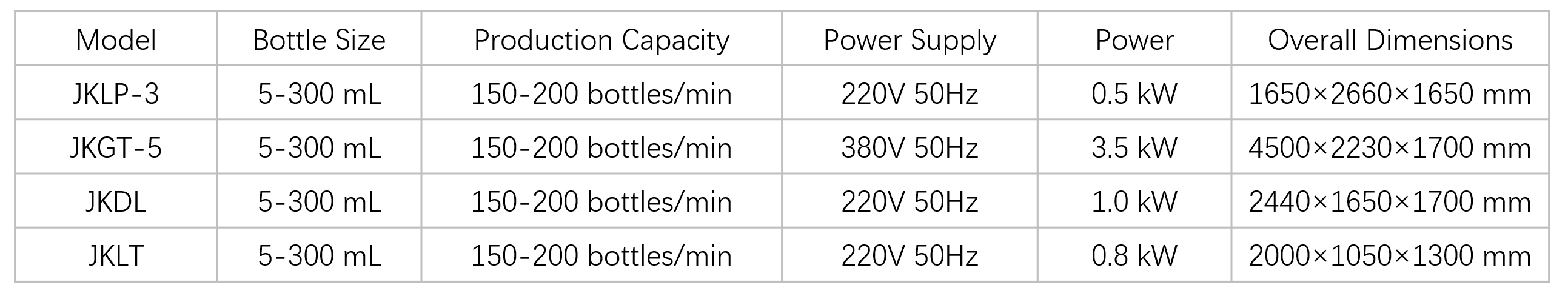
(verified under 100 mL plastic bottles, syrup product conditions)Parameters
Process Flow
Bottle infeed → Air cleaning (compressed air + vacuum dust removal, 16 blow–suction nozzles) →
Filling (±1% accuracy, servo-controlled piston pumps) → Capping (continuous rotary type with slip protection, acceptance rate ≥99%) → Labeling (accuracy ±1 mm) → Cartoning (up to 220 cartons/min)
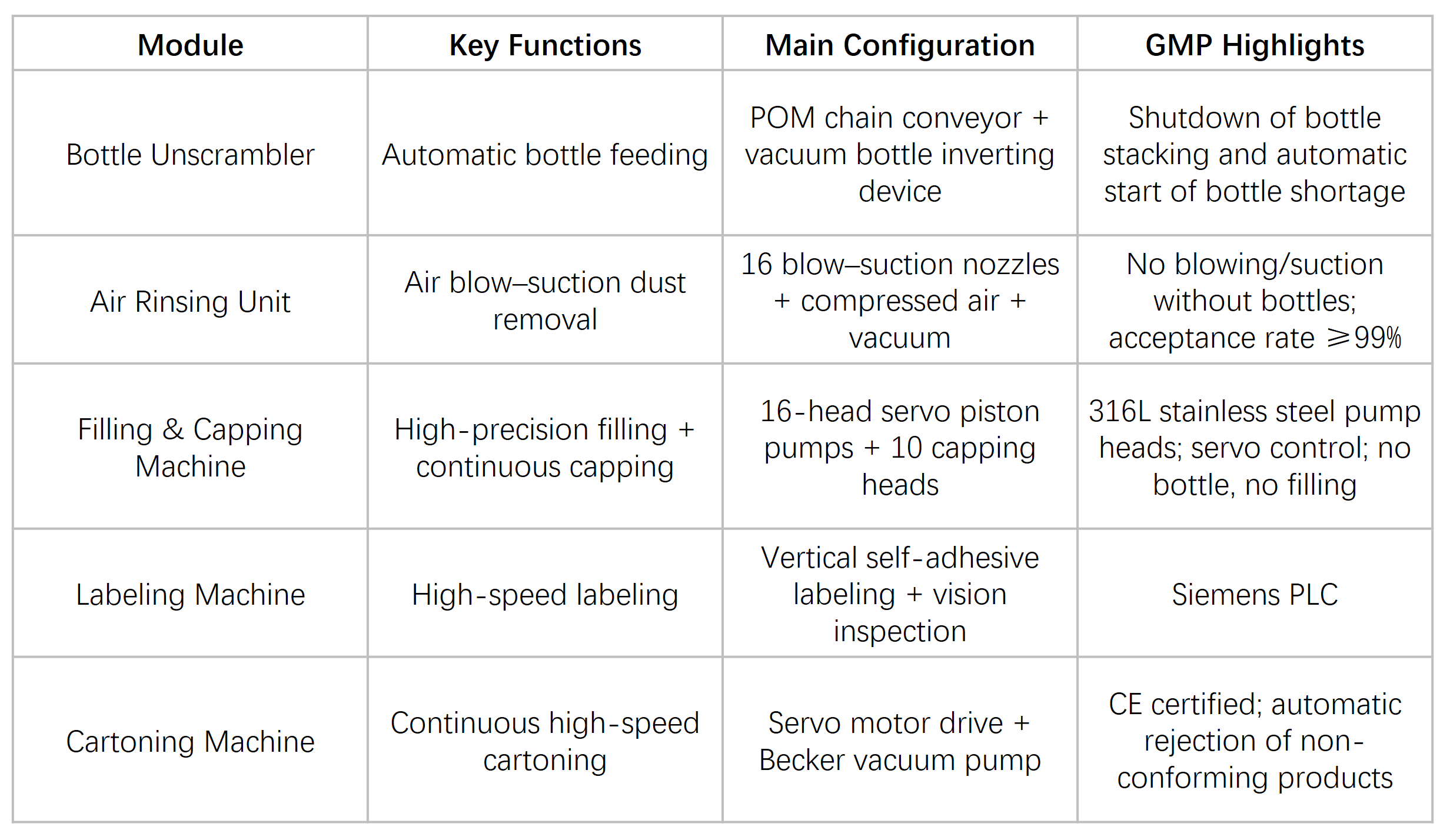
Core Modules
Control System & Data Integrity
The entire line is controlled by a Siemens PLC with industrial touch-screen HMI, ensuring stable module coordination and easy operation. Sensors cover critical conditions such as missing bottles, missing caps, and line full status, guaranteeing safe and reliable operation.
The system supports multiple recipe presets and one-touch changeover, with automatic recording and export of operation logs and alarm history to meet GMP and audit traceability requirements.
MES integration interfaces (Modbus / OPC) are reserved, and an optional electronic batch record module is available to support digital production management.
Cleaning & Sterilization Strategy
Product-contact parts of the filling system support CIP (Clean-in-Place), achieving ≥99% cleaning coverage with no residual dead zones.
Buffer tanks, filling pumps, and valves are made of 316L stainless steel, fully compliant with GMP cleanliness requirements.
All modules are designed for easy disassembly, allowing efficient manual or in-place cleaning.
Utility Requirements


GIVE US A MESSAGE