XWZ300 Intelligent High Speed Cartoner
BRIEF INTRODUCTION
Standalone Machine Positioning
Independently performs carton opening, leaflet folding, cartoning, batch coding, carton sealing, and reject functions, meeting GMP requirements for standalone production.
Applicable Packaging Types & Specifications
Applicable products:
Blister packs
Vials
Ampoules
Ointment tubes
Injection trays
Rectangular products
Flat products
Production capacity: 100–300 cartons/min
Process Flow
Manual / automatic feeding → Machine process: carton picking → carton opening → leaflet folding → product loading → leaflet insertion → batch coding → carton sealing → rejection → Carton discharge
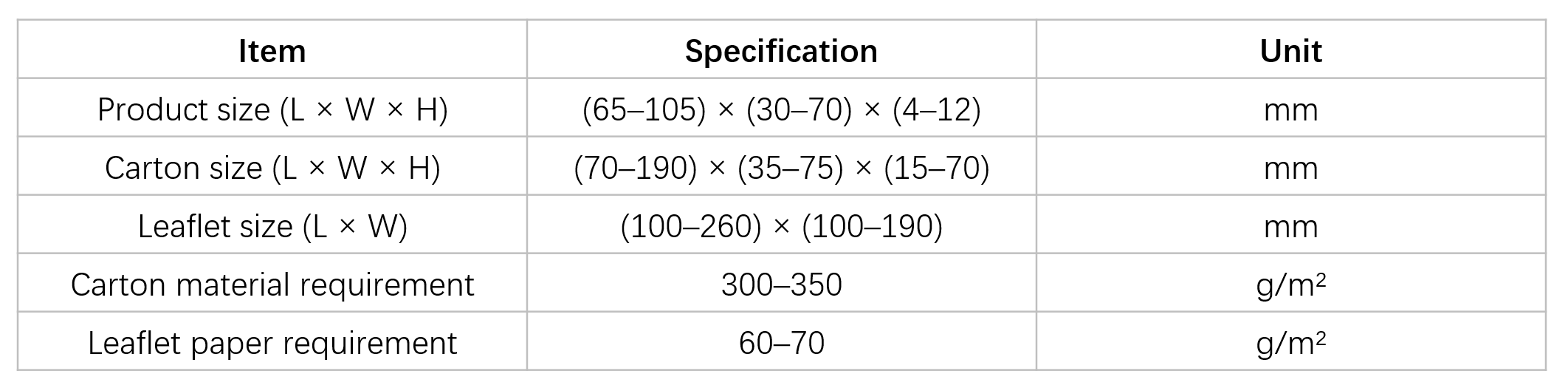
Key Technical Parameters
Structure and GMP Design
The machine adopts a balcony-style structure with a fully stainless steel body, and features enclosed electrical and pneumatic circuits. The drive unit is positioned at the rear, leaving the operator side fully open. The suspended design facilitates collection and cleaning of debris, aligns with ergonomic principles, complies with GMP requirements, and allows convenient operation and maintenance.
The equipment is enclosed with a 304 stainless steel casing and aluminum-alloy tempered glass protective doors, equipped with CE-certified Schneider door magnetic switches. Opening the protective door automatically stops the machine, ensuring operational safety.
Horizontal continuous cartoning design, with suction cups feeding boxes in coordination with a rotating chain moving in the same direction; multiple heads and stations simultaneously load products into boxes. A linear cam mechanism ensures product loading without impact, with smooth operation, minimal noise, and reduced load.
The machine is equipped with a mechanical overload automatic protection device. Product changeovers do not require part replacement; handles with adjustable set screws allow tool-free adjustments. All parts in contact with products are made of stainless steel and non-toxic materials, fully complying with GMP requirements.
Control System
The system integrates mechanical, electrical, optical, and pneumatic automation, achieving fully automated pharmaceutical packaging. Each operation stage is equipped with advanced detection methods, and non-conforming products are automatically rejected to prevent human error and avoid product contamination, meeting GMP standards.
A human-machine interface (HMI) touchscreen allows adjustment of programs at each workstation, production status monitoring, and fault/alarm display.
The PLC fully controls the program, working in tandem with optical detection systems to automatically identify and reject insufficiently filled blister packs, carton jams, and other non-conforming products, ensuring consistent product quality. Automatic counting data is synchronized and displayed on the screen.
The main motor adopts variable frequency drive (VFD) control, allowing flexible adaptation to different production requirements.
Utility Requirements

GIVE US A MESSAGE