PW System
BRIEF INTRODUCTION
Standalone Machine Positioning
Designed to independently perform Purified Water (PW) preparation, fully meeting GMP requirements for standalone production.
Applicable Capacity & Specifications
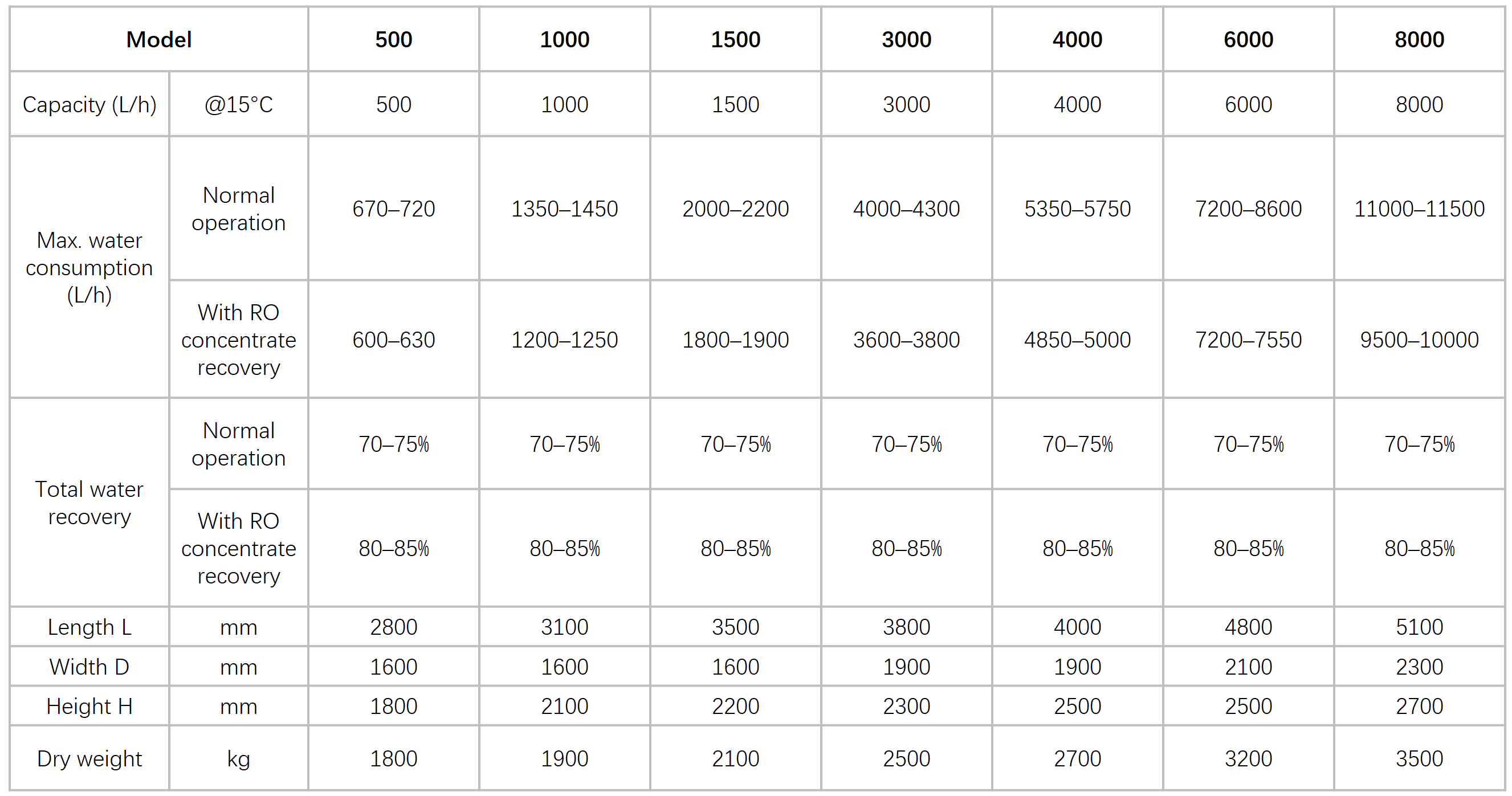
Capacity range: 250 L/h – 10,000 L/h
(Measured at feed water temperature of 15 °C)
Process Flow
Raw water supply → In-system processes: pretreatment (quartz sand / activated carbon / softening) → Reverse Osmosis (RO) with multi-stage filtration & desalination → EDI (Electrodeionization) → purified water meeting pharmacopeia standards → Water outlet
Product Model Parameters
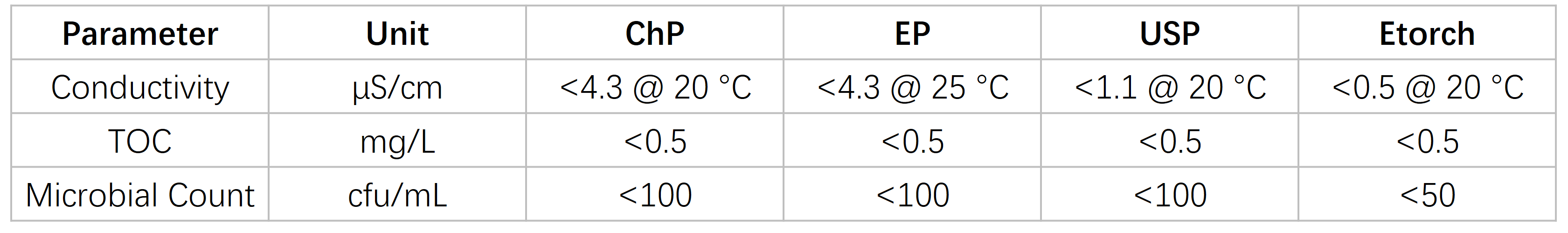
Complied Pharmacopeia Standards
Features & Advantages
Capacity: 250–10,000 L/h per unit, with customizable system configuration.
Microbiological Control: Advanced feedwater pretreatment combined with fully automatic pasteurization ensures stable low bioburden levels.
Energy Efficiency & Zero Liquid Discharge (ZLD): Compact modular design with closed-loop recirculation and zero liquid discharge, equipped with remote monitoring and control capability.
Utility Requirements

RO System
RO+EDI System
Hot Water Disinfection Purification Water System
Second Class RO+EDI Purified Water System

我们的产品
GIVE US A MESSAGE










