Non-PVC Film Soft Bag Filling Line
BRIEF INTRODUCTION
Product Positioning
Designed to meet GMP-compliant, fully automated production requirements for aseptic large-volume parenterals (LVP), covering the complete process from film roll feeding → bag forming → filling → sealing → bag discharge.
Applicable Packaging & Specifications
Packaging type: Non-PVC multilayer film bags
Volume range: 50 – 2,000 mL
Peak capacity: 600 bags/hour
(tested with 500 mL bags containing aqueous solution)
Process Flow
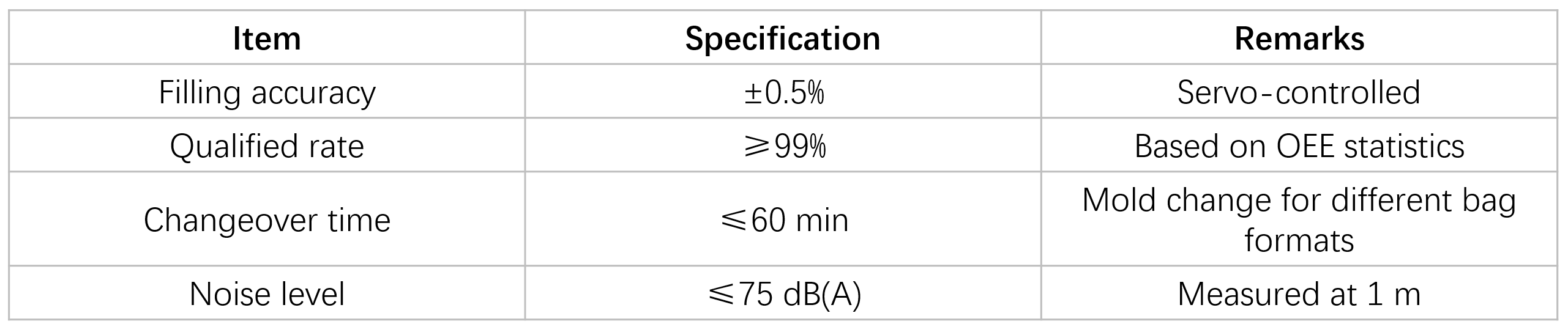
Material feeding → Machine operations: thermal transfer printing → bag forming → port welding → filling (accuracy ±0.5%) → sealing (non-contact, dust-free) → Bag discharge
Key Performance Parameters
Control System & Data Integrity
Siemens PLC + industrial touchscreen; supports 21 CFR Part 11 audit trail.
Recipe management, ≥100 sets, one-click switching.
Batch record generation, electronic signature, PDF export, MES integration (via OPC UA).
Cleaning / Sterilization Strategy
Fully automatic CIP/SIP, filling system sterilized online at 121 °C without component replacement; residual removal complete.
Cleaning coverage ≥99%, no residual liquid; all connecting parts made of 316L stainless steel, corrosion-resistant and high-temperature resistant.
Non-toxic hoses and gaskets, withstand up to 125 °C without particle shedding.
Utilities Requirements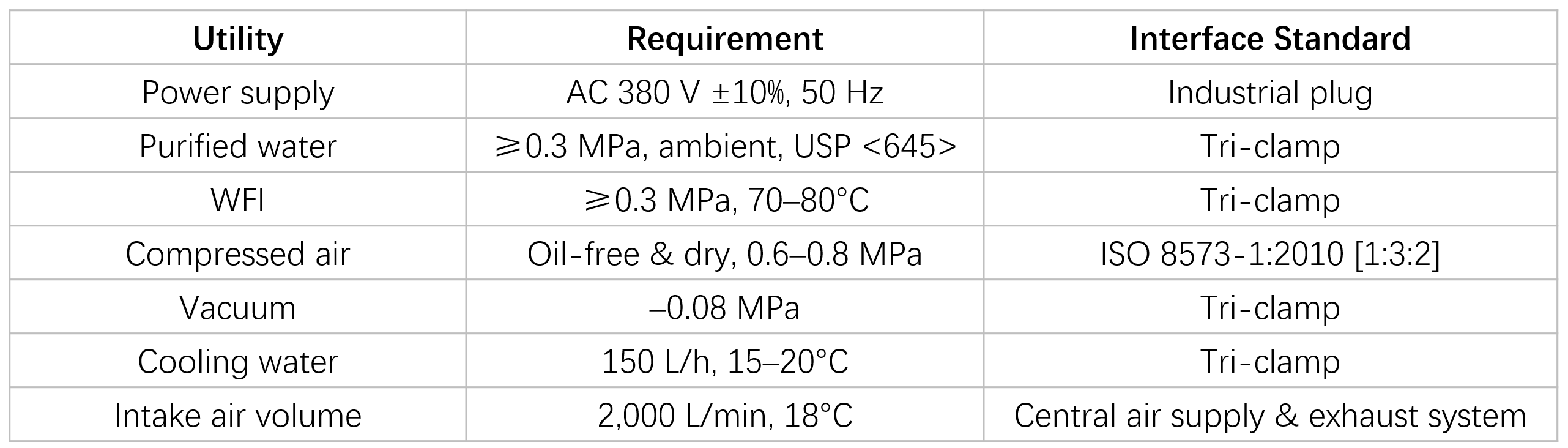
GIVE US A MESSAGE





