Preparation System
BRIEF INTRODUCTION
Standalone Positioning
Independently completes the compounding (liquid preparation) process, fully meeting GMP requirements for standalone production.
Applicable Dosage Forms & Specifications
Dosage Forms: Lyophilized powder for injection / aqueous injection vials, infusion bottles, prefilled syringes, liposomes, ADCs, vaccines, etc.
Batch Volume: 50 – 10,000 L
Design Capacity: ≤10,000 L per batch, with continuous buffer discharge up to 2,000 L/h
Process Flow
Manual / Automatic Charging → Integrated Process of This Unit: Vacuum Conveying → Raw & Auxiliary Material Feeding → Weighing → Dissolution → Mixing → Temperature Adjustment → Sterilizing Filtration → Dynamic Buffering → Aseptic Transfer → Aseptic Transfer to Downstream Filling Machines
Key Performance Parameters
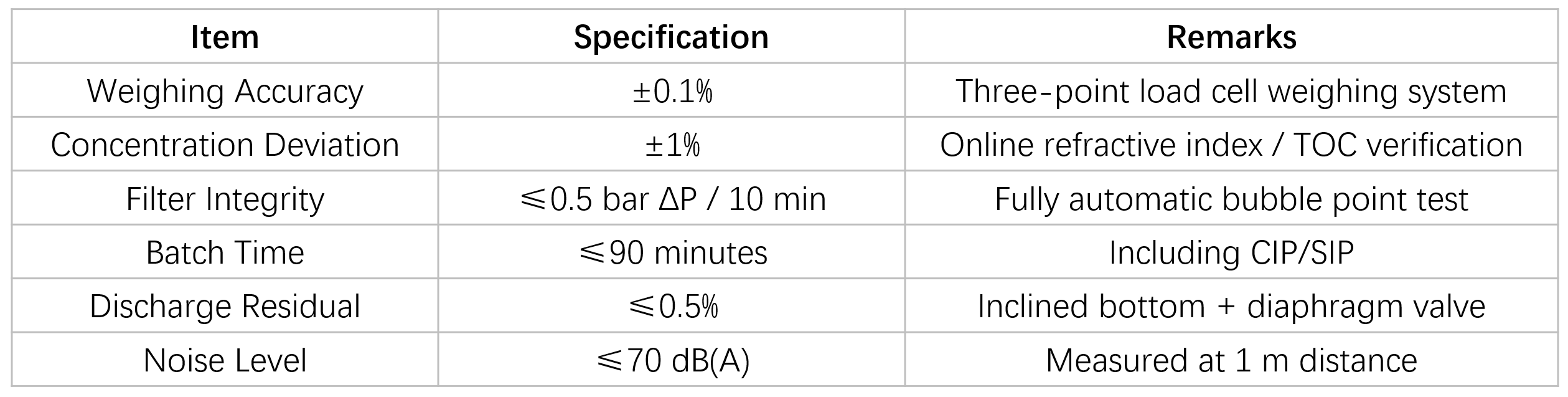
Construction & GMP Design
316L stainless steel three-dimensional frame, electropolished to Ra ≤ 0.4 μm, with sloped surfaces ≥3° and zero dead legs.
Fully enclosed RABS enclosure with tempered glass viewing window; all seals made of USP Class VI EPDM/PTFE.
Fully modular piping assembly with 100% TIG automatic welding and endoscopic inspection.
Jacketed/heatexchanger dual-circuit design with temperature control ±1 °C; can be integrated with CIP station and WFI loop to achieve a closed-loop “self-cleaning – self-sterilization” process.
Control System
Siemens S7-1500 PLC + 15" HMI, ≥100 recipes with hierarchical user access management.
21 CFR Part 11 compliant: audit trail, electronic signatures, one-click PDF batch report generation.
Reserved OPC UA and Modbus-TCP interfaces for seamless integration with MES / LIMS and Electronic Batch Records (EBR).
Optional AI-assisted formulation optimization, based on historical concentration–yield big data.
Utility Requirements

GIVE US A MESSAGE



